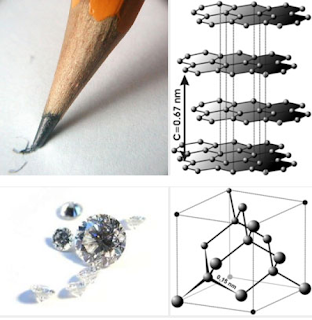
 |
| Graphite vs. diamonds, Dept. of Geosciences, Penn State University |
***
It appears that diamonds have been the subject of research around the world for quite a while. And the results coming in say it's not the hardest substance there is and, possibly, not even that rare. As you know, diamond is a form of carbon, the major chemical constituent of most organic matter.In the U.S. Johns Hopkins University reports Diamonds May Not Be So Rare As Once Thought. Reasons why they are still rare:
For one thing, the prevalence of diamonds near the Earth’s surface – where they can be mined – still depends on relatively rare volcanic magma eruptions that raise them from the depths where they form. For another, the diamonds being considered in these studies are not necessarily the stuff of engagement rings, unless the recipient is equipped with a microscope. Most are only a few microns across and are not visible to the unaided eye.Reason why they are not rare:
The new research showed that water could produce diamonds as its pH falls naturally – that is, as it becomes more acidic – while moving from one type of rock to another, Sverjensky said.
The finding is one of many in about the last 25 years that expands scientists’ understanding of how pervasive diamonds may be, Sverjensky [Johns Hopkins geochemist] said.
“The more people look, the more they’re finding diamonds in different rock types now,” Sverjensky said. “I think everybody would agree there’s more and more environments of diamond formation being discovered.”Meanwhile in other labs substances have been discovered and created that are harder than diamond: wurtzite boron nitride and a cubic form of boron nitride and the mineral lonsdaleite; nano-rods of carbon (ACNR); fullerite; and q-carbon.
Of these q-carbon is the most intriguing because it's easy to make, is magnetic at room temperature, and glows when a voltage is applied. Here are links to 4 articles about q-carbon: ExtremeTech, ScienceNews, Live Science, and a more scholarly article from AIP (American Institue of Physics).
To get an idea of the various forms carbon can take, have a look at Wikipedia's article, Allotropes of carbon.
-- Marge

No comments:
Post a Comment